Immunization committee to recommend provinces suspend AstraZeneca use among those under 55: sources
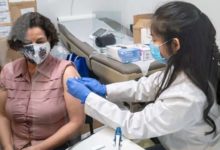
Canada’s National Advisory Committee on Immunization (NACI) is expected to recommend today a pause in the use of the AstraZeneca-Oxford COVID-19 vaccine on those under the age of 55 because of safety concerns, sources told CBC News.
P.E.I. suspends AstraZeneca vaccine program
The updated guidelines will be issued later today, according to sources who spoke on the condition of anonymity. The expected change in guidance comes following reports of rare blood clots in some immunized patients.
Canada is expected to receive 1.5 million doses of this product from the U.S. on Tuesday.
The AstraZeneca shot has not been widely used in people under the age of 55 in this country. Some jurisdictions, such as P.E.I., have been using some of their supply to immunize young people who work in public-facing sectors like grocery and convenience stores.
A spokesperson for P.E.I.’s health department confirmed delivery of the vaccine had been suspended.
“Appointments at pharmacies for AstraZeneca vaccine for those 18-29 are on hold pending anticipated further information from Health Canada and NACI,” the department said in an email.
This is just the latest issue the company has faced over the last three months.
Earlier this year, a number of European countries halted vaccinations in response to questions about the AstraZeneca product’s efficacy in people over the age of 65, only to restart them after new evidence emerged.
After Health Canada approved the shot for all adults, NACI recommended the product be used only on people under the age of 65, citing a dearth of clinical trial data on the vaccine’s effectiveness in older people.
NACI changed course earlier this month after reviewing three “real-world studies,” saying the two-dose viral vector vaccine can and should be used on seniors.
The European Medicines Agency also has had to assure European Union member countries that the product is safe to use in response to reports of post-vaccine blood clots in a very small number of patients.
The agency concluded that the benefits of protecting against COVID-19 — which itself results in clotting problems — outweigh the risks.
The Public Health Agency of Canada has said it’s “possible” the vaccine may be associated with “very rare but serious cases of blood clots associated with thrombocytopenia” — a condition associated with very low levels of blood platelets. Health Canada has maintained that the benefits of the AstraZeneca COVID-19 vaccine continue to outweigh the risks.




Redes Sociais - Comentários